Describe the Relationship Between Hydrogen Ions and Ph
AS SOON AS POSSIBLE. O pH and hydrogen ions are independent.
Relationship Between Hydrogen Ions And Ph Definition Properties Ph Scale And Values
The relationship between the pH and H3O concentration is described by the equation pHlog10H3O where H3O is the concentration of the hydronium ions.
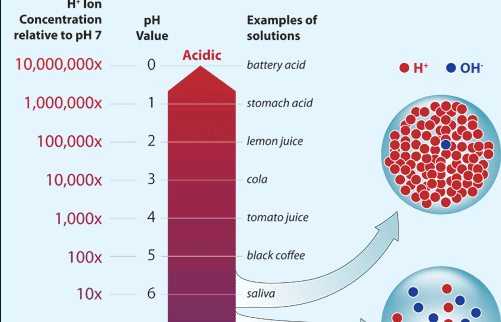
. The degree of acidity or basicity can be. O pH is always 100 times more than the number of hydrogen ions. PH is the measure of the hydrogen ion concentration of a solution.
O pH and hydrogen ions are inversely proportional. Acids and Bases Numerically. April 04 2022 The pH changes based on the concentration of hydrogen ions in a substance.
In an acidic solution the concentration of hydrogen ions depends on the concentration or molarity of the acidic solution. The hydrogen ion concentration decreases by a factor of 10 so the pH increases by 1 from 16 to 26. Describe the relationship between dissolved carbon dioxide and ocean pH 3.
PH measures the H ion concentration of a solution which depends on where or not an acid or base was dissolved connect exothermic endothermic An exothermic chemical reaction releases more energy than it absorbs. Then the pH is the logarithm of the inverse of the hydrogen ion concentration. PH pOH 14.
Concentration of hydrogen ions increases pH value decreases. Compare the hydrogen ion concentration in vinegar to window cleaner. Over 7 Describe the relationship between the hydrogen ions H and pH.
Describe the relationship between the Hydrogen Ions H and pH. Relate hydrogen ion concentration to pH. A solution with a concentration of hydrogen ions higher than 10-7molL is acidic and a solution with a lower concentration is alkaline another way to say basic.
Not all solutions are neutral when this happens the hydrogen ion concentration is greater than the hydroxide ion concentration and is known as an acidic solutionWhich means that H is greater than 10 x 10-7 M. O pH and hydrogen ions are equivalent. Using this expression one can determine the pOH if the pH is known.
Compounds with high pH readings are basic or alkaline whereas those with. Describe atomic structure and function. Describe the relationship between pH and hydrogen ion concentration.
Relate the characteristics of water to processes critical for life. PH -log 10 a H Where a is the activity. Testing pH Lab Background A liquid may be an acid base or neutral.
Solutions with high concentration of hydrogen ions have a low pH and are acidic. Solutions with a low concentration of hydrogen ions have a high pH and are basic. Question A solution of hydrochloric acid with a concentration of 2 gdm 3 has a pH of 13.
Higher molarity of acid lower pH value. From this equation we can see that an increase of hydrogen ions will lower the pH and a decrease of hydrogen ions will raise the pH. Differences in pH are in orders of ten in relation to the hydrogen ion concentration.
A solution at pH 55 would thus have a. Explain how ocean acidification affects marine life. Using the formula pH-logH a pH of 7 is neutral a pH less than 7 is acidic and a pH greater than 7 is.
Richard Nelson Date. Which means that H is. This increase in hydrogen ions is what decreases the pH.
When pH is at 70 the concentration of hydrogen ions and hydroxide ions is the same. As pH decreases below 70 the hydrogen ion concentration increases and hydroxide ion concentration decreases. In acidosis urine pH may fall as low as 45 due to excess H Renal tubules increase rate of H secretion elevating pH In alkalosis as high as 82 because of excess HCO3 Renal tubules decrease rate of H secretion and allow neutralization of bicarbonate lowering pH.
O pH and hydrogen ions are directly proportional. PH -log 10 H aq Therefore there is a direct relationship between pH and the concentration of. Perhaps the most important connection between pH and concentration is that pH is by its very nature a measure of the concentration of hydrogen ions in a given solution.
1The pH of a solution describes its acidity or alkalinity. This is the result of the sum. In addition some of the hydrogen combines with carbonate to form more bicarbonate.
Describe the relationship between hydrogen ions H and pH. Hydrogen ion concentration is inversely related to pHthe higher the hydrogen ion concentration the lower the pH and the more acidic the solution. There is an important inverse relationship between pH and pOH in that pOH values greater than 7 define solutions that are acidic.
Describe how pH and H3O concentration are related and explain why diluting an acid raises the pH but diluting a base lowers the pH Answer. An acid with a higher molarity will have a higher concentration of hydrogen ions and hence a lower pH value. How is pH related to a solutions acidity.
Hydrogen ion concentration and thus a decrease in pH. A solution with the pH 3 is 1000 times less acid than a solution with the pH 1. As pH increases above 70 the hydrogen ion concentration decreases and hydroxide ion concentration increases.
Since the concentration of the hydrogen ions is often very low ion activity is considered as equal to the concentration of hydrogen ions. A base is a solution with a larger hydroxide ion concentration than the hydrogen ion concentration. Use this page to check your understanding of the content.
Relationship Between Hydrogen Ions And Ph Definition Properties Ph Scale And Values

The Ph Scale Chemistry For Non Majors

Small Drop In Ph Means Big Change In Acidity Woods Hole Oceanographic Institution
No comments for "Describe the Relationship Between Hydrogen Ions and Ph"
Post a Comment